What is Nuclear Fallout?
Nuclear fallout is dust and dirt (of varying sizes) that's been thrown up into the air by the force (blast) of a nuclear explosion, and has become radioactive because of the nuclear reactions inside the explosion. "Radioactive" means that it is giving off radiation (a much better explanation will come on this page in the future, here is a one-sentence one.) Many tons of earth are thrown up high into the air, and they fall back down to the ground at varying speeds (which depend on their size/weight and the wind conditions).
The amount of fallout depends a lot on how the nuclear bomb is exploded. If the bomb is an "air burst" there will be only minimal fallout, while a "ground burst" explosion (meaning the explosion happens on the ground) will create a lot of fallout.The fallout particles from a ground burst consist of the dust as described above.
The fallout from an air burst consists of very small particles, those that are already up there in the air, which have then been made radioactive due to the nuclear explosion. These very light particles will stay high up in the air a long time, and only gradually come down to earth over a huge area (perhaps much of the planet). By this time their radioactivity has greatly diminished (see graph below). This, and the dilution from being spread over a vast area means that the danger from fallout from an air burst is drastically less than that from a ground burst explosion.
A general rule with radioactive substances (like fallout) is that the "hotter" the material is (meaning the faster it's emitting radiation), the quicker it takes for the level of radiation to decay (that is, to reduce towards safe levels). The word "hot" is used in this sense to mean how radioactive something is, which is nothing to do with its actual temperature in the usual sense of the word. Fresh nuclear fallout particles are extremely hot, which means they are extremely dangerous when first created, but they decay quite rapidly. This decay is shown in the graph below.
The actual chemical makeup of fallout is quite complex, as there are several radioactive isotopes involved, and each of these decays (i.e. changes into another element as it emits radiation) into several different isotopes, so it can get quite complicated in terms of chemistry.
In fact fallout from nuclear fission in a bomb contains a very complex mixture of over 300 different isotopes of 36 different elements. If you're interested in this see here on Wikipedia for more info. About 60 grams of fission products are produced for each kiloton of fission energy yield, or 60 kilograms per metagon. This fission energy yield is all of the yield of a purely fission bomb (an "atom bomb" or "A-bomb), and only part of the yield of a thermonuclear bomb (a hydrogen bomb or H-bomb). Meaning that the more powerful bombs will give less fission products (which make up nearly all of the fallout) overall. [Reference: Effects of Nuclear Weapons paragraph 9.12-9.15]
An isotope is an atom of a particular atomic mass (i.e. weight). For example there are three naturally occurring isotopes of Hydrogen, H-1, H-2, and H-3. Uranium has the isotopes U-235 and U-238, and others less well known. The numbers refer to how heavy each atom of it is compared to one ordinary hydrogen (H-1) atom. They also equal the total number of protons plus neutrons in the atom's nucleus. Chemistry and electronics are concerned with interactions between the electrons in atoms. Here we are discussing nuclear explosions and nuclear radiation, which comes from the nucleus of atoms. Each type of isotope has it's own unique and distinctive type of nucleus.
Note also that the different isotopes in nuclear fallout all have different decay rates. The ones which are the most radioactive (or "hot") decay the fastest, so they are initially the most dangerous, but they decay to a less (or not) dangerous state relatively fast.
Nuclear Fallout from Slowly Decaying Isotopes
Isotopes with really slow decay rates (e.g. those which you sometimes hear about with half lives of billions of years) are decaying so slowly that only a low rate of radiation is given off from them. The half-life is how long it takes for half of the original substance (isotope) to change into a different substance (isotope) as it emits radiation. If it takes a very long time to decay, that means it's decaying very slowly. Because radiation is emitted as a part of radioactive decay, that also means that it's emitting radiation very slowly (compared to faster decaying isotopes).
These slowly-decaying radioactive isotopes are not that much of a worry at the small amounts present in nuclear weapons. Nuclear power reactors may be another story as the total amount of nuclear waste that's already been generated in nuclear power reactors adds up to about 80,000 tonnes. Which is something like 1000 times more than the fissile material in all the world's 4000 active nuclear warheads combined. (Though its composition and distribution are also very different.)
Using a high estimate (see the next section below) of 5000 megatons for the total world's active nuclear arsenal, an estimate of half of that yield being from fission (as opposed to fusion which makes no fallout) and 60 kilograms of fission products per megaton of fission yield (from above), gives a total of 150 tonnes of fission products in total from an all-out nuclear war. 150 tonnes is a lot different to 80,000 tonnes. (Though its composition and distribution are also very different.)
Nuclear Fallout from Medium-Term Decay Rate Isotopes
Some isotopes with medium-term decay rates can become dangerous after a while as they can accumulate in food, such as Strontium-90 with a half life of 29 years. These are not radioactive enough to kill people quickly, but some of them can accumulate in living tissues (Strontium-90 can replace calcium and accumulate in bones, causing cancer).
There have been claims that this will result in the death of everything on Earth, however there have already been about 500 above ground nuclear bombs detonated, and we are nowhere near killing everything on the planet just from this effect alone. Here you can see real photographs of American cities with nuclear bombs (from tests) exploding on the horizon. According to Wikipedia, the peak of worldwide nuclear radiation from above ground testing was 0.15 millisieverts per year (this is similar to 0.015 R per year, referring to the units used further down on this page). That was in 1963, and it made up 7% of the average background level of radiation from all sources. Since above ground testing was banned in 1963, this has decayed to 0.005 millisieverts per year, which is about 0.0005 R per year. For comparison, the natural background dose (which is around us all the time, and has been more or less since the Earth was formed) is about 2 millisieverts (or 0.02 R) per year.
Based on this is seems extremely unlikely that detonating even 8 times as many nuclear bombs as have already been exploded above ground (approximately the entire world's total deployed nuclear weapons) would permanently destroy everything on the planet from fallout.
Other reports of more localised effects of fallout (such as from nuclear testing) are not as optimistic, although even these are affecting only parts of the world, not the whole world. For example, a study showed that children born in St. Louis, Missouri in 1963 had levels of strontium-90 in their deciduous teeth that was 50 times higher than that found in children born in 1950, before the advent of large-scale atomic testing [source link]. Commentators on the study said that the fallout was likely to cause increased cases of diseases in those who absorb strontium-90 into their bones. This is of course very bad, but not nearly equivalent to destroying all of life on Earth.
According to this page (and the previously mentioned one which use the same source), strontium-90 is probably the most dangerous component of nuclear fallout. This page claims that probably the most serious threat is cesium-137, a gamma emitter with a half-life of 30 years (similar to strontium-90 which is 28 years). The same page does a more sophisticated version of the calculation I made above on the ratio of effects of fallout from a full scale nuclear war compared to the existing nuclear test data, and predicts an additional "about 1/2 percent to 15 percent of the estimated peacetime cancer death rate in developed countries". Which again is very bad, but not the end of life on the planet. And the estimate they used of 10,000 megatons of bombs seems quite high given there are only 4000 active nuclear warheads, nearly all of which are much less than a megaton — and seems more related to the 1980s when there were tens of thousands of nuclear warheads (before arms reductions treaties greatly reduced the numbers).
In the estimates in the above paragraph, I get the feeling they're talking about danger to large areas or to the world as a whole. This is supported by a quote from the book Effects of Nuclear Weapons, which says on paragraph 9.43, "At one time it was suggested that the explosion of a sufficiently large number of nuclear weapons might result in such an extensive distribution of the plutonium as to represent a worldwide hazard. It is now realised that the fission products — the radioisotope strontium-90 in particular — are a more serious hazard than plutonium is likely to be."
From Wikipedia:
As of 1993, worldwide, 520 atmospheric nuclear explosions (including 8 underwater) have been conducted with a total yield of 545 megaton (Mt): 217 Mt from fission and 328 Mt from fusion, while the estimated number of underground nuclear tests conducted in the period from 1957 to 1992 is 1,352 explosions with a total yield of 90 Mt.
I had a quick look for a good estimate of how many megatons are in the world's current actively deployed nuclear arsenal, but didn't find a good answer. This page says 5000 megatons, but I think this is referring to inactive and active nuclear weapons combined. Since that same page says there are 1800 nuclear weapons on high alert. Each weapon is on average much less than a megaton. The total for the USA in 2012 could be calculated from this table, which shows the numbers of all of the different types of nuclear devices in the US nuclear arsenal. Each type of device can be looked up on the internet, e.g. here, to see its yield in megatons. For example there are currently 100 active and 520 inactive B83 bombs, each with 1.2 megatons, which is (I think) the largest current US nuclear device. There are 752 active W76 warheads which are 100 kilotons (0.1 megatons) each. These W76 warheads are the most numerous in the current US arsenal, and are mostly (or entirely) carried by Trident Submarine-Launched Ballistic Missiles (SLBM).
Even a total yield of 5000 megatons is only nine times the total of 545 megatons that we've already detonated in the atmosphere. This again suggests very strongly that an all-out nuclear war would not mean anything remotely like the end of all life on Earth.
Note also that most of the yield of most modern bombs (i.e. hydgrogen bombs a.k.a. thermonuclear bombs) comes from fusion, not fission. It is fission products which make up nuclear fallout. The ratio of fission to fusion yield given in the quote above for the total of world atmospheric testing is probably approximately similar to the overall ratio of fission to fusion yield in a nuclear war. Which means it's realistic to compare the overall yields for past tests and current stockpiles and get a rough idea of how much more fallout would be released in a nuclear war than in the total of previous atmospheric tests.
From the estimates above, it seems realistic to estimate that detonating all the current actively deployed nuclear weapons would result in about 10 times, or less, than the amount of fallout that has already been released in atmospheric nuclear tests.
Perhaps the main factor not considered here is the height of detonation above ground. If the explosion happens higher up, there is less fallout. Assuming that the range of heights covered in nuclear tests is simliar to that which would be used in an actual war (which seems like a reasonable assumption although may not be correct), these estimates are still fully valild.
I'll look into this further but it seems logical that in more localised areas the much shorter lived decay products (like a few hours or days) will be by far the most damaging to life, since the rate of radiation released by them greatly exceeds that of the mid or long-term fission products.
Nuclear Fallout from Short-Lived Isotopes
This graph is for fallout overall, i.e. the combined effect from all the different isotopes which make up nuclear fallout. However in the initial stages the overall amount of radiation is dominated by the short-lived isotopes, since these are the ones which both emit radiation the fastest, and decay (into something else) the fastest.
Most people viewing this graph for the first time will probably be quite surprised as to how quickly the levels of radiation decay.
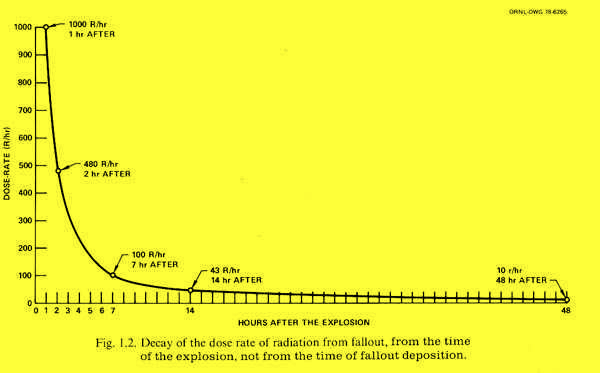
Decay of the radiation from nuclear fallout (Source: Nuclear War Survival Skills)
Notice that the horizontal scale on this graph is in hours after the explosion. As you can see, even after 14 hours, the danger level has dropped dramatically to only 1/20th of the level one hour after the explosion. By 48 hours (two days) the radiation level has reduced to 1/100th what it was at one hour after the explosion.
The vertical scale of the graph above is the dosage rate (i.e. the level of radiation) measured in röentgens per hour (R/hr). The "röentgen" is a unit of radiation dose and is pronounced "RENT-gehn" (or sometimes "RONT-gehn") with the accent on the first syllable "rent", pronounced like the rent you might pay to a landlord, and the second syllable (that could be written as "gehn" or "gen", with the "g" as in "gun" and the "en" as in "pen". The röentgen is a unit of accumulated radiation dose, so röentgens per hour correspond to the dose rate. A radiation dose of around 100-200 röentgens is likely to cause recoverable radiation sickness, and a few hundred röentgens or more is likely to cause death. As the accumulated dose climbs into the 500-1000 range and above, death becomes certain.
Since the scale of the above graph is in röentgens per hour, you can figure out approximately how long it would take to accumulate an incapacitating or fatal dose at different times after the explosion. Even the "low" dose of 10 R/hr at the far right of the graph (after 48 hours) is still quite dangerous. At a steady dose of 10 röentgens per hour, in 48 hours (two days) you would accumulate 480 röentgens — which could easily be a fatal dose. And much more than that (say double) would certainly be fatal.
At the levels of fallout shown in the graph, it would take about two weeks (approximately) before the radiation has reduced to a safe enough level to be out of a shelter permanently. Though after two days, at 10 R/hr you could go outside for short periods (even for a couple of hours) if needed to perform essential tasks (such as getting water or whatever) without too much worry, since 1-2 hours outside would mean 10-20 R of exposure, which is not going to kill you. In areas of really heavy fallout you may need to be in your shelter longer than that, and in many areas (probably most areas not too close to ground zero or directly downwind) it would be less than two weeks.
If you live far enough away (perhaps a few hundred kilometres, perhaps less, depending on wind direction etc.) from any ground burst nuclear detonations you are unlikely to need a shelter at all. Although this may be different (i.e. you may still need a shelter) if a really large number of warheads, like hundreds or thousands, are detonated — such as may happen in the northern hemisphere — even if you are a long way away. A device that can monitor the levels of radiation is the only way to really know when it is safe or when you need to be in a shelter.
Consider also that the time scale on the graph is measured in hours after the explosion itself. So unless you are very close to ground zero, the fallout will have already decayed during the time it takes to get to your location. (e.g. in the time from 1 to 2 hours after the explosion the fallout rate has halved). This and other factors (such as wind) mean that the actual levels of radiation from nuclear fallout may be less than shown in the graph.
Note that there is much less transfer of air (due to wind currents) between the Northern and Southern Hemispheres than there is from east-west or west-east within each hemisphere. Nearly all the warheads in a nuclear war would be detonated in the Northern Hemisphere. This means that in the Southern Hemisphere, we are much safer from nuclear fallout that spreads over a really large area as would occur in a global nuclear war. There is a region of very limited wind near the equator, called the Intertropical Convergence Zone (ITCZ), and known by sailors as the doldrums.
It's possible for boats to get trapped in this zone for days or weeks. The region was extremely dangerous in the days when wind-powered sailing boats were the only means of international transport, and there was no radio equipment to signal distress, or motor-powered rescue vehicles.
In terms of nuclear war survival, however, the zone is a blessing for the Southern Hemisphere — as it means nuclear fallout from the North will take much, much longer to get down here. According to the ABC, "There is some exchange between the two [hemispheres], but the process takes a year or two, versus about a week for air to circulate within a hemisphere." When you consider how the radioactivity of fallout decays over time, this is a massive advantage for those who live in the South.
Units
(This section is fairly technical and isn't really needed for nuclear war survival, except for perhaps the one sentence in bold, three paragraphs below, which says that for nuclear fallout, the three "R" units basically all the same thing.)
There is an almost ridiculous seeming variety of different units used to quantify levels and doses of radiation; such as röentgens, rems, rads, curies, becquerels, grays and sieverts. This can get pretty confusing so, for those interested in the numbers, I'll try to simplify it.
Most of the Cold War vintage literature uses the röentgen as the main unit of radiation dose. By "dose" I mean a total amount of radiation that's accumulated over a period of time, as opposed to a rate or strength of radiation level. Because most of the Western-world information on nuclear weapons was released during that time period, I've got used to using the röentgen. Röentgen is sometimes spelt without the first "e", i.e. röntgen.
Conveniently, the other two units which begin with the letter "r" are all basically equal to each other in terms of dose absorbed by people or animals. The unit rem is the "röentgen equivalent in man" and for "men", or even women, it works out pretty much equal to a röentgen. The rad is also basically the same for this subject. So, without getting too technical, when you see the letter "R" you can think of any one of these units — röentgens, rems, or rads — which for our purposes are all close enough in value to mean the same thing. The abbreviation R is officially meant to stand for röentgens, but is often used to mean rems, and perhaps rads also. One roentgen will deposit about 0.96 rem (so pretty close to 1 rem) in soft biological tissue. Where the radiation is dominated by gamma or x rays applied uniformly to the whole body, (which is the case for nuclear fallout), 1 rad of absorbed dose gives 1 rem of effective dose.
The different units were created because radiation can be counted in different ways. It can be counted as the amount of energy (e.g. in joules) absorbed, or by the effects on living tissue, or by the degree that air is ionised (electrified) by the radiation, or the rate that individual atoms emit particles of radiation. The röentgen is based on how much air is ionised, which was the easiest to measure in the old days of nuclear physics (from about the 1930s). The rem is based on how much effect there is on tissue. The rad (short for "radiation absorbed dose") is based on joules of energy absorbed per kilogram of matter. A sievert is 100 rems and a gray (symbol Gy) is 100 rads. The other units are the the curie and becquerel which are based on how many actual nuclear decays there are per second — since nuclear radiation occurs when the nucleus of an atom changes itself spontaneously (i.e. "decays") into either another type of element or a lower energy state, and gives off some other high-energy particles and/or electromagnetic radiation when it changes. The exact conversions between all these units will depend on what atoms are decaying (i.e. are radioactive), what type of radiation, and what type of material is absorbing the radiation. Here on Wikipedia is some more information on all the different units used for radiation.
What You Need to Do About Nuclear Fallout
If you consider that a dose of any more than say 100 röentgens is going to be a problem (and ideally you want a lot less than that), you can see on the graph that in the initial stages of high fallout, shortly after a nuclear explosion, there is going to be a problem. In order to survive high levels (or even moderate levels) of nuclear fallout, you will require some sort of protection — such as a fallout shelter.
The second nuclear war survival fact that may surprise most people is that these shelters do not have to be elaborate underground fortresses. Basic home made shelters that can be made in a few days (or even several hours if sufficiently prepared) can provide adequate shielding from nuclear fallout. These are commonly known as "expedient" shelters, with the word expedient meaning "quick and easy". Of course an elaborate professionally constructed underground bunker is going to be much more comfortable. But if you don't have one of those available, there are alternatives which are much easier to construct.
The key to understanding fallout (other than learning about the decay rates) is that the fallout itself is not highly poisonous in the usual sense (its mostly just ordinary dirt although this does not mean you would want to eat it), but each grain of dirt is emitting deadly gamma rays which have massive penetrating power, and travel in straight lines right through most objects and out the other side.
It's these gamma rays that are the main danger from fallout, especially in the early stages soon after the explosion, when the radiation levels are the highest.. I'll expand on this much more soon but basically you need 2-3 feet of earth (or anything weighing about the same as that) to block the vast majority of the gamma rays emitted by nuclear fallout. There are also alpha and beta particles but these are much less of a concern in the short term (meaning days, weeks, and months) than the gamma rays. Over many years the accumulated dose from intermediate-length-decay isotopes like strontium-90, which beta-decays, can become a problem.
Gamma rays have a lot of penetrating power, it takes 9 centimetres of packed earth (or anything weighing the same as that) to halve their intensity. Every 9cm of earth between you and whatever is emitting the gamma rays (e.g. the fallout dust that's covering much of the land outside your shelter) will halve the intensity. So with 18cm of earth you have 1/4 the intensity, 27cm will allow 1/8 of the radiation through, and so on. If you don't know your 9 times table you can think of it as 10cm if you like since its close enough. 90cm is 9 x 10, so 90cm of packed earth is 10 halving thicknesses. So the amount of radiation that you would get is reduced to 1/2 x 1/2 x 1/2 x1/2 x 1/2 x 1/2 x 1/2 x 1/2 x 1/2 x1/2 of what it would be outside. Which can also be written as as (1/2)^10, or 1/(2^10), in math speak you would say that as "one half to the power of ten", or "one over two to the power of ten". 2^10 is 1024, so that equals 1/1024, or about 1000th of the gamma radiation will penetrate 90cm of earth.
This is the type of thickness you want ideally in a fallout shelter in areas of really high fallout. Though any protection at all will be an improvement on no protection. And many areas will not have enough fallout to need that much shelter (or even no shelter if you're far enough away from any nuclear bomb detonations, depending on wind and other conditions).
Food and Water
From "Nuclear War Survival Skills": If the fallout particles do not become mixed with the parts of food that are eaten, no harm is done. Food and water in dust-tight containers are not contaminated by fallout radiation. Peeling fruits and vegetables removes essentially all fallout, as does removing the uppermost several inches of stored grain onto which fallout particles have fallen. Water from many sources — such as deep wells and covered reservoirs, tanks, and containers — would not be contaminated. Even water containing dissolved radioactive elements and compounds can be made safe for drinking by simply filtering it through earth, as described later in [the] book.
Other things to avoid are eating meat from diseased animals, or eating organ meat from animals which have themselves eaten large amounts of fallout.
The Long Term Effects of Exposure to Nuclear Fallout
Assuming you survive the initial exposure, the long-term effects of exposure to nuclear fallout are much less than what you probably think. I'll add more to this later..... But studies done from real-world situations (e.g. Hiroshima) have shown that only a very small fraction of people exposed to nuclear bomb radiation (even at close to fatal levels) have suffered from serious long-term or delayed effects.
Surviving the collapse of modern society would require a mix of practical skills, knowledge, and mindset. Here are some of the most important skills: Basic Survival Skills: ...
A Nuclear Strike On One Single City
Due to the speed at which nuclear war is expected to escalate, an isolated nuclear attack on just one single city is probably somewhat unlikely. Here we consider a fictitious scenario assuming such an attack...
Kallan and the Thunder Beast
The Encounter In a lush, untamed world where the ancient and the primeval collide, a young warrior named Kallan prowled the dense forest. His long hair, knotted and wild, cascaded down his back, a testament to...

 Welcome to Prepping.com.au, the new web magazine.
Welcome to Prepping.com.au, the new web magazine.
 What is Prepping? A guide for normal people to get started.
What is Prepping? A guide for normal people to get started.
 How to use this site. When all else fails, read the instructions.
How to use this site. When all else fails, read the instructions.
 This is your mission, should you choose to accept it.
This is your mission, should you choose to accept it.









